While a lot of things that can cause muscle cramps, and there can be many contributing factors that make you more at risk for muscle cramps. It’s important to know what can make you more susceptible to muscle cramps, and how we can prevent muscle cramps. Let’s start at the beginning and dive in to discover what causes muscle cramps
What Causes Muscle Cramps?
The primary cause of muscle cramps is the overuse of a muscle. Think of it as a “ticking time bomb” waiting to go off. The first thing to address is the overuse, because it is the primary cause. Overuse can look a couple of different ways, it can be both overuse from moving the muscle a lot, or overuse from holding the muscle in the same position. Either way, putting strain on the muscle can lead to cramps. Once you ID the movement or position, that will help you solve your muscle cramp problems.
What Are the Underlying Causes That Make Cramps a “Ticking Time Bomb”?
- Blood Supply Issues: if you have inadequate blood supply in your limbs, and most especially your extremities, severe cramps can occur. If these cramps are caused by blood supply, then they should go away fairly quickly after you stop exercising the muscle.
- Nerve Compression: Affecting seniors and those with back problems, nerve compression in the spine (lumbar stenosis) can create cramps in the legs that gets more and more painful after walking. To ease the pain, walk slightly hunched–as though you were pushing a shopping cart. Then consult your doctor.
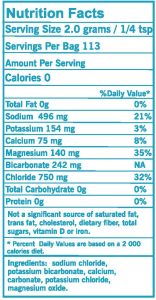
- Mineral Depletion or Electrolyte Loss: There are minerals that are essential to making sure that your muscles and nerves are operating effectively, and these are called electrolytes. While the electrolyte we hear about most often is sodium, there are other minerals that are equally important, such as potassium, calcium, magnesium, and bicarbonate. Sweating will deplete these electrolytes, but so will frequent urination, diarrhea and vomiting, and diuretics (medicines prescribed for high blood pressure). Replenish all those minerals with our Boulder Salt.
What Puts You More At Risk For Getting Muscle Cramps?
There are some medical conditions that make you more prone to getting cramps, some of which you can control and some of which you can’t. These are:
- Age: The older you get, the less effective your muscles are at, well, everything. This is just a fact of aging. With aging muscles, your muscles can wear out sooner during athletic events and begin to cramp.
- Dehydration: Hydration should ideally replace electrolytes, but even if you don’t have access to electrolytes like Boulder Salt, getting plenty of water is a good way to stop the onslaught of muscle cramps.
- Pregnancy: Pregnancy is hard enough by itself, but it also comes with the problem of increased muscle cramps.
- Medical Conditions: Diabetes, nerve disorders, liver disorders, and thyroid problems can all increase your chance of getting muscle cramps.
How Can I Prevent Muscle Cramps?
- Hydrate: Drinking a lot of water before and during a physical event is not enough to prevent muscle cramps. You should be regularly hydrating your body so your muscles are used to being fully hydrated–not just during periods of athletic activity. The amount of water you should drink depends on your height and weight, your sex, the level of activity, the heat (heat can really exacerbate muscle cramps), your health and age, and the medicines you take. Being good about always drinking water should be the first thing you do.
- Replace Electrolytes: Replacing electrolytes while you’re doing physical activity is essential. Some people say that the best way to replace electrolytes is through sports drinks, and it’s true that some sports drinks have the necessary minerals needed. But not all sports drinks do, and more often than not the minerals are accompanied with a lot of sugar. A better way to replace electrolytes is to use Boulder Salt, which contains all of the electrolytes you need, and putting this salt in your water will be a tasteless solution–you won’t feel like you’re drinking salt water, just good pure water.
- Stretch: Stretching your muscles before and after physical activity can be extremely effective. Even if you get muscle cramps when you’re not doing physical activity, such as while you sleep, you should stretch a little before and after bed. This stretching doesn’t need to be extreme, but a few minutes on a stationary bike, or a little light exercise, should be enough to prevent muscle cramps most of the time.
All of these factors will help you ease muscle cramps or prevent them from happening in the future! Shop our selection of Boulder Salt today!